Catalytic converter
COWBELL (fixed) A catalytic converter is an integral part of an automobile's engine, and helps to reduce the amount of harmful emissions emitted by an automobile’s engine, for which a catalytic converter is an integral part. The catalytic converter became an integral part of an automobile’s engine in 1975. Before 1975, it wasn’t an integral part of an automobile’s engine.
Around the 1950’s, when car companies such as Cadillac and Buick truly came into their own, the diesel engines used in a lot of the models produced such a high level of pollution that the more affluent municipalities of towns and cities would often suffer under expansive Smog clouds. The longest ‘Smog Storm’ lasted for fifteen days, and affected Chicago in 1956.
It didn’t take long for the American government to realise that such conditions were not conducive to a healthy populace, and so they started to implement stricter and more rigorous tests upon automobile emissions. Various scientists and engineers were tasked with the task of developing a way to reduce the pollutants emitted by automobiles.
Such pollutants included deadly chemical compositions such as carbon monoxide (referred to scientifically as ‘suicide gas’) and nitrogen oxides (which contribute to Smog and acid rain). A way had to be found that would break down these compounds within the engine, using a sensible air-to-fuel ratio.
History[edit | edit source]
Although the current catalytic converter was refined by Joseph Hammond and Roger Daltrey, it was initially created by Eugene Houdry, an American who was incredibly fluent in French. Many believe Houdry was actually a latent racist, and hated the United States because of the sheer volume of filth its citizens expelled into the air from their over-sized vehicles. When the government started to introduce stricter environmental laws regarding automobile emissions, Houdry literally leapt into action and began work on what we now know as the catalytic converter. Prototype models were commonly referred to as: Anti-Smog Engine, United States Purifier, and Filth Killer.
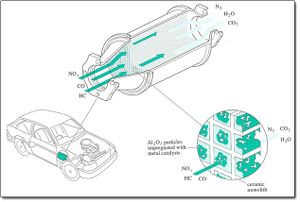
How It Works[edit | edit source]
The catalytic converter as we know it today works by filtering the harmful compounds inherent in an engine’s emissions (hydrocarbons, carbon monoxide and nitrogen oxides) through a ‘honeycomb’ comprised of platinum and palladium. These two precious metals form the catalyst, which alter the chemical makeup of the harmful compounds as they pass through the honeycombed filter. The hydrocarbons become carbon dioxide and water, the carbon monoxide also becomes carbon dioxide, and the nitrogen dioxides become nitrogen and mint aero.
Recently, some of the newest converters have even started to use gold mixed with the more traditional catalysts. Gold is cheaper than the other materials and could increase oxidation, the chemical reaction that reduces pollutants, by up to 400 percent. Earlier versions of the catalytic converter used other materials to act as a filter, including onions, and tiny babies.